
the
evidence
is
limited
and
results are
sometimes based on
experimental
or
in
vitro models
only
[11].
Constituents
of
cigarette
smoke,
such
as polycyclic
aromatic hydrocarbons
(PAH),
require metabolic
activation,
evasion
or
detoxifica-
tion
processes,
and
subsequent
binding
to
DNA
to
exert
their
carcinogenetic
action.
Consequently,
mutations
or
functional polymorphism
in genes
involved
in PAH metabo-
lism and detoxification may modify and
influence
the effect
of
smoking
on
PCa
pathogenesis
[50] .The
glutathione-S-
transferases (GSTs) comprise a class of enzymes that detoxify
tobacco-related
carcinogenesis
including PAHs by
conjugat-
ing
glutathione
to
facilitate
their
removal.
Of
the
seven
mammalian
GST
classes
characterized,
those
that
have
shown
substrate
specifically
for
PAH metabolites
and
that
are
expressed
in
the
human
prostate
include
GSTP
and
the
GSTM.
Human
prostatic
epithelium
predominantly
expresses
the GSTP1
subtype,
and
loss
of GSTP1
expression
has
been
observed
as
one
of
the
earliest
events
in
prostate
carcinogenesis
[51].
In
an
exploratory
case–control
study
evaluating
122
cases
of
PCa
and
122
controls,
among
participants with
the
genotype GSTP1
Ile/Ile,
smoking was
associated with
an
increased
risk
of
PCa with
an
adjusted
odds
ratio
(OR)
of 4.09
(95% CI, 1.25–13.35)
compared with
nonsmokers
[51].
GSTM
variants
have
also
been
shown
to
affect
the
association
between
smoking
and
PCa
risk.
In
a
family-based case–control design
study
(439 PCa cases and
479
brother
controls),
among white
participants
(90%
of
the
study
population),
heavy
smoking
increased
PCa
risk
nearly
twofold
in
those with
the GSTM1 null genotype
(OR:
1.73;
95%
CI,
0.99–3-05),
but
this
increased
risk was
not
observed
in
heavy
smokers
who
carried
the
GSTM1
nondeleted
allele
(OR: 0.95%; 95% CI, 0.53–1.71)
[50]. Mu-
tation
in
the
p53
gene,
one
of
the most mutated
tumor-
suppressor
genes
in
human
neoplasms,
or
in
the
human
cytochrome
P450,
which
is
involved
in
the
metabolic
pathways
of
several
endogenous
and
exogenous
com-
pounds
as
steroids
and
environmental
xenobiotics, may
play
a
significant
role
in modifying
PCa
risk
in
smokers
[52] .Increased
hemeoxygenase
1
(HO-1) messenger
RNA
expression
and
upregulated HO-1
protein
levels,
induced
by
smoking,
are
also
present
in
PCa
cell
lines
[53] .HO-1
may
have
a
role
in
tumor
angiogenesis
[54] .Exposure
to
carcinogenic
substances
found
in
cigarettes
(eg,
cadmium) has been
proposed
as
an
alternative mecha-
nism
for
PCa
carcinogenesis.
Cadmium
has
been
shown
to
indirectly
induce
prostate
carcinogenesis
through
interac-
tion with
the androgen
receptor. Ye et al
[55]have
reported
that cadmiumcan activate the androgen receptor response
in
human
PCa
cell
lines
in
the
absence
of
androgen
but
in
the
presence of
the
androgen
receptor. Cadmium also
enhances
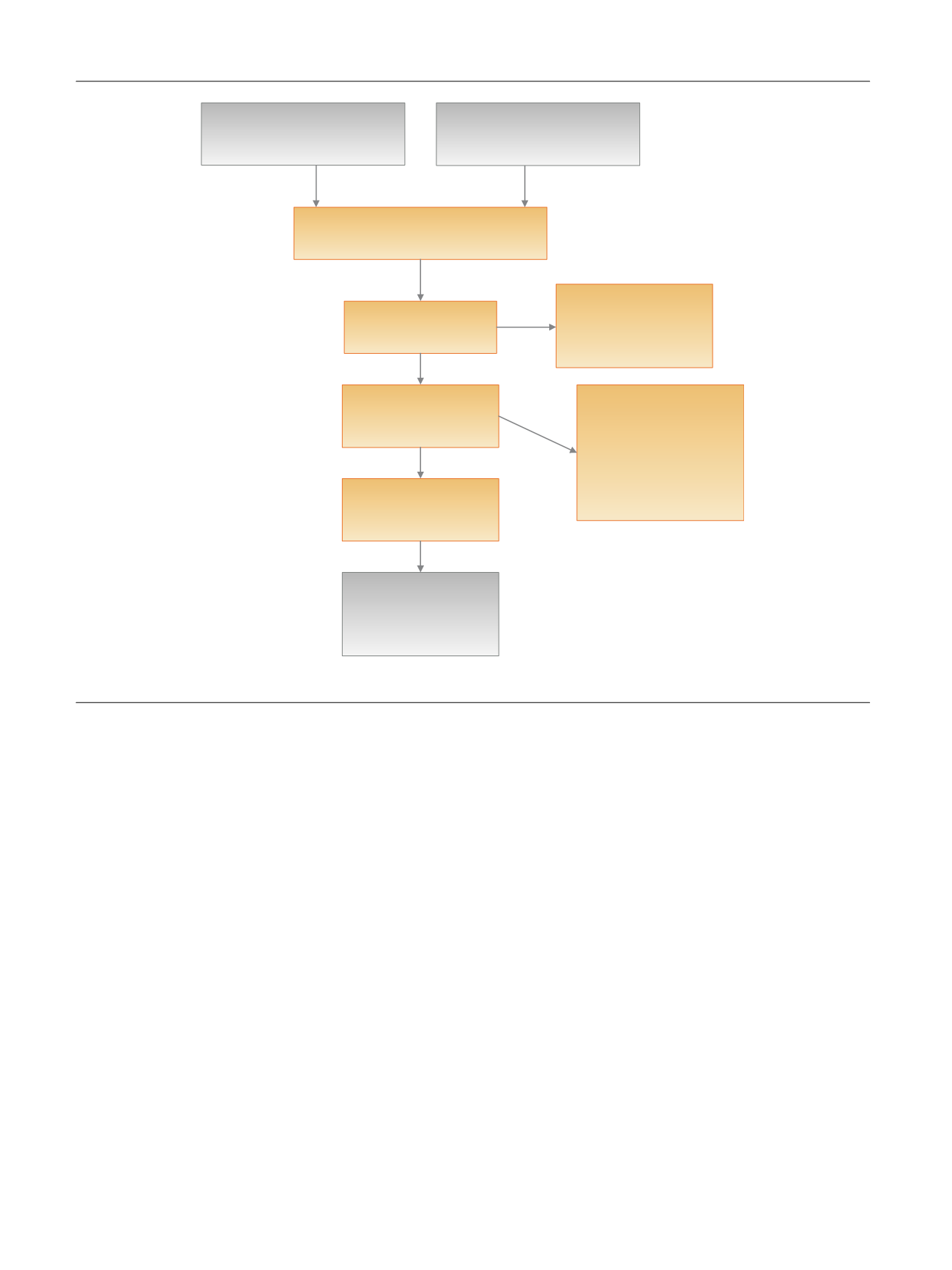
Records idenƟfied through
database searches
(
n
= 792)
AddiƟonal records idenƟfied
through other sources
(
n
= 10)
Records aŌer duplicates removed
(
n
= 554)
Records screened
(
n
= 248)
Records excluded because
they did not meet our
inclusion criteria
(
n
= 146)
Full-text arƟcles assessed
for eligibility
(
n
= 102)
Full-text arƟcles excluded
•
Similar or more
informaƟve data from the
same study were available
in another arƟcle (
n
= 25)
•
Not a systemaƟc review
(
n
= 9)
Studies included in
qualitaƟve synthesis
(
n
= 37)
Studies included as
addiƟonal references
(
n
=
31)
Fig.
1
–
Flow
diagram
of
the
search
results.
E U R O P E A N
U R O L O G Y
F O C U S
1
( 2 0 1 5
)
2 8 – 3 8
30

















